Those who suffer hip pain will consider just about any treatment to get their mobility back including hip replacement surgery. A National Institutes of Health (NIH) article reports each year that 332,000 total hip replacements are performed in the United States. Vast improvements in technology for artificial hip replacement parts has allowed them to withstand more stress and strain and last longer – thus benefitting younger, more active people as well as those over 60.
However, some hip replacement parts are causing more pain than relief, specifically, the Stryker Rejuvenate Hip Implant System. In 2010, the Stryker Corporation, one of the world’s largest medical device companies operating in the world, released the Rejuvenate Hip Implant System; a modular hip system of femoral bodies and necks. According to the company, the Rejuvenate Hip Implant System was designed to provide the following potential:
- Enhanced Stability
- Proven Modularity
- Intraoperative Flexibility
In 2009, The Stryker Company touted the Rejuvenate Modular Primary Hip System benefits to surgeons with the following assurances:
“[U]nparalleled options for personalizing the implant to each patient’s anatomy. The Rejuvenate System is designed to optimize anatomic restoration by providing options that offer enhanced stability, proven modularity an intraoperative flexibility. The modular design enables the surgeon to independently manage stem size, leg length and offset to recreate the patient’s anatomy, restore biomechanics and, consequently, minimize the risk of dislocation.”
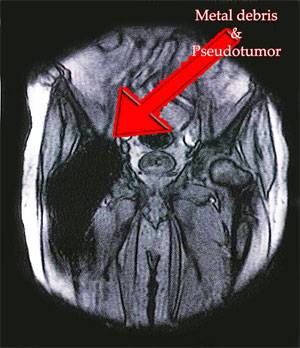
The Rejuvenate Hip Implant System stem bodies are made of TMZF alloy, a proprietary Stryker material with a plasma sprayed coating of commercially pure (CP) titanium and PureFix HA. The modular necks are made of CoCr alloy. While the Rejuvenate Modular Hip System is not a metal-on-metal hip system, there have been increasing reports of metal injuries at the location where the metal modular stem meets the metal neck of the device.
These injuries may show that the Rejuvenate Modular Hip System is defective and causing harm to those who have already been implanted with the new device. As a result, Stryker Rejuvenate lawsuits are being reviewed throughout the United States. While the Rejuvenate Hip Implant has not been recalled in the United States its global distribution has been halted as of 2012. Source: Reuters
Problems after Hip Replacement Surgery? Time to Discuss your Stryker Hip Replacement Case.
Because many hip replacement patients are unaware of the type of implant used, cases are being investigated for any individuals who have experienced problems after a surgery since 2008. If you or someone you know has received the hip implant component, suspected they may have or are suffering from the following:
- Unexplained hip pain more than three months after hip replacement
- Loosening of their artificial hip implant
- Hip replacement revision surgery
Contact Babbitt & Johnson, P.A. to schedule a free, no-obligation case evaluation. The Stryker Hip Litigation Lawyers are currently reviewing potential litigation involving the Stryker Rejuvenate Modular Primary Hip System. Call us at (561) 375-2841.